-40%
Medical Digital Precision Optical Pupilometer PD Meter Ophthalmic Equipment AA
$ 66
- Description
- Size Guide
Description
Medical Digital Precision Optical Pupilometer PD Meter Ophthalmic Equipment AADescription:
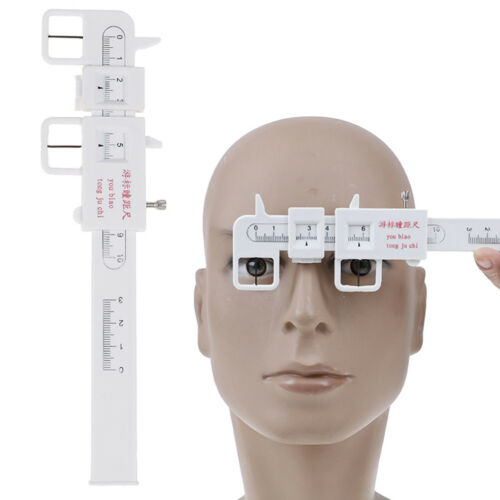
PD Meter Is a Digital Precision Optical Instrument Used to Measure Distance Between Human Pupils in The Process of Optometry.
Features:
It Features Consecutive Measurement, Direct Point-Sampling and High Accuracy of Orientation, With Mechanical Hairspring Being Used to Level at The Reflecting Point of Human Cornea.
It Adopts Linear Sensors of High Precision, Highly Intellectualized Electronic System and Digital Display of Measurement Results Which Is More Accurate, Direct and Easy to Read.
The Design of LED Lamp-House and Low Power-Consumption Makes The Battery Life Prolonged.
The Brightness of LED Is Adjustable.
+2.0d Compensation for The Degree of Eyesight Is Available.
Technical Parameter:
Range of Measurement: Binocular Pupillary Distance: 45~82 Mm
Left or Right Pupillary Distance: 22.5~41 Mm
Indication Error: ≤ 0.5 Mm
Rounding Error: ≤ 0.5 Mm
Distance of Target: 30 Cm~∞
Power Source: Voltage: DC 3v
Specification:
Power: AA Battery (not Include)
Time for Automatic Shut-off:
About 1 Minute After Stopping Operation, or Turn It off Manually.
Size: As pictures shown
Weight: 0.68kg
Package Include:
1 X Digital PD Meter
Statement:
FDA Disclaimer:
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(The seller's name: Helen Lee,City:Beijing , State: Beijing, Country: China, Telephone number: 86-18796658874)
This item has been cleaned and treated according to the manufacturer's instructions.
About Warranty:
We guarantees new equipment other than accessories to be free from defects in workmanship and materials for a period of
24 months
from the date of shipment under normal use and service.
Return Policy:
1. If you have a defective item which you want to return, please contact us
within 30 days
from you receive the item.
2. We will refund the money to you when we get the return items. Or replace item for you.
GOOD NOETS : Above 200$ ,we normally send the item via express delivery,such as UPS ,DHL ,TNT ,EMS ,safe and fast,normally take 7-10 business days to worldwide.
W
e
commit ourself to do 100% customer satisfactione .Thanks very much.
1).The trade is cross-border and Airmail is the cheapest post way, so it take a long time for delivery.
2).We are not Responsible for your Customs duty .
3).We don't add taxes, VAT or other hidden charges.
We accept payment by
PayPal
. PayPal enables you to make payment quickly and securely online.
1: We only accept payment via PayPal.
2: Please pay the money within 5 days after the auction ended.
3: Please offer home or mobile phone when the item's Value exceed 100(USD,EUR,AUD,GBP)
We are the manufacturer and distributor of medical equipments for more than 10 years.If you are interested in the distribution work, please feel free to contact us.
If you have any question,please feel free to contact us
before you open case or leave us negative feedback
,we'll do our best to help you .W
e
commit ourself to do 100% customer satisfactione .
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health.